Horsehair worm manipulates mantis with its own genes

Horsehair worms, which live parasitically in various insects during their larval stage, drive their host to suicide. Tappei Mishina and colleagues wondered how they acquired the potential to make this happen.
A striking and gruesome example of parasites that manipulate their host are horsehair worms. During their larval stage, they live in crickets, grasshoppers, and mantises, but as adult worms they live freely in water. To get there, they drive their hapless host to commit a self-destructive act: it jumps into the water. Horsehair worms can disrupt the behaviour of their host so dramatically thanks to genes they picked up from it, Tappei Mishina and colleagues show.
In water, adult horsehair worms (Nematomorpha) mate in a knotted mass of males and females; that is why they are also called Gordian worms. The females then lay eggs from which microscopic larvae hatch. In order to develop further, they must move to insect hosts that live on dry land. The hosts can ingest the larvae directly with their food or via a ‘transporter’, for instance a mayfly. Living in water during its larval stage, this insect is exposed to horsehair worm larvae. The adult mayfly flies out and may be grabbed by an insect host, which then becomes infected with a parasitic horsehair worm larva.
Horror
And then, a horror story starts. The horsehair worm larva grows into an extremely thin worm that can reach several times the length of the host. By the time the parasite matures, it forces its host to behave unnaturally. The host, no longer in charge of himself, starts wandering until it comes across water. Then it enters the water body, often with death as a result. If it survives, it will be infertile.
But the worm is in its element. It wriggles out of the insect’s body and starts looking for conspecifics. If the host is attacked by a predatory water insect before the worm is out, it will emerge more quickly. And if the host is swallowed by a fish or frog, the worm manages to escape from that fish or frog also.
How can horsehair worms so dramatically manipulate the behaviour of their hosts, from which they differ greatly from an evolutionary perspective, Mishina wondered.
His research on mantis Tenodera angustipennis and horsehair worm Chordodes fukuii shows that the worm literally took over the biochemistry of its host.
Expression pattern
The researchers first examined which genes are activated or deactivated in the horsehair worm and in the mantis brain, and how this pattern changes during host manipulation. They show that only in the worm does the expression pattern change: during manipulation, many genes are read and transcribed to be translated into proteins that were previously inactive, while other genes are silenced. The worm produces proteins to influence the praying mantis’ brain, is the conclusion.
They then compared genes from Chordodes species with information about known genes and proteins stored in databases. This yielded a surprising result: more than 1,400 genes of the parasites are very similar to genes of mantises. Especially these genes are expressed differently during manipulation; most are more strongly activated, others are suppressed. Other horsehair worm species than Chordodes species, which have other hosts, do not possess these mantis genes.
Horizontal gene transfer
It seems that Chordodes has picked up genes from its hosts, mantises, over the course of its evolutionary history – and not just a little. That happened not once, but many times. It is not surprising that the proteins encoded by these genes have an effect in mantises.
Gene transfer between animal species, which is called horizontal gene transfer, is a special and, as far as we know, very rare phenomenon. The researchers suggest that it may also play a role in other cases of host manipulation.
Willy van Strien
Photos: ©Takuya Sato
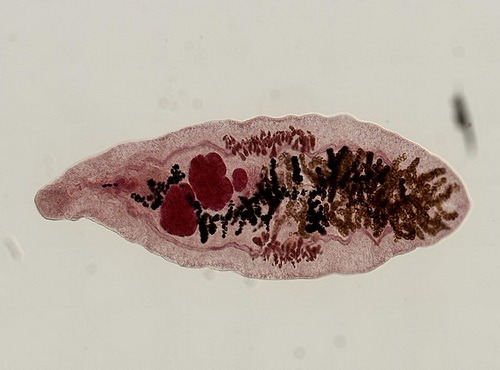
Large: mantid Tenodera angustipennis
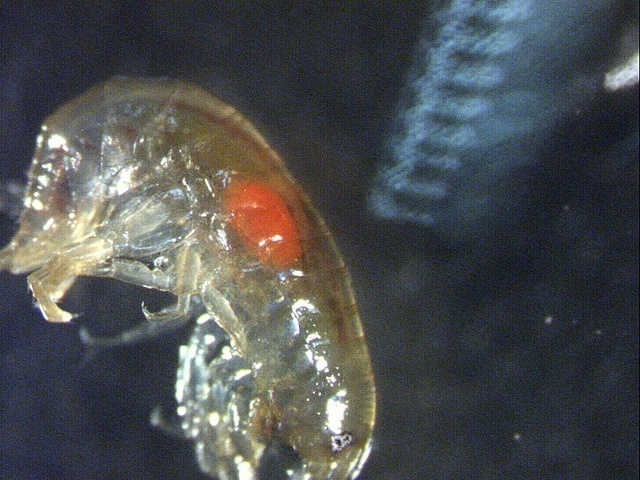
Small: mantid Tenodera angustipennis and Chordodes horsehair worm
A horror video with horsehair worms on YouTube
Sources:
Mishina, T., M-C. Chiu, Y. Hashiguchi, S. Oishi, A. Sasaki, R. Okada, H. Uchiyama, T. Sasaki, M. Sakura, H. Takeshima & T. Sato, 2023. Massive horizontal gene transfer and the evolution of nematomorph-driven behavioral manipulation of mantids. Current Biology, online 19 October. Doi: 10.1016/j.cub.2023.09.052
Sánchez, M.I., F. Ponton, D. Missé, D.P. Hughes & F. Thomas, 2008. Hairworm response to notonectid attacks. Animal Behaviour 75: 823-826. Doi: 10.1016/j.anbehav.2007.07.002
Ponton, F., C. Lebarbenchon, T. Lefèvre, D.G. Biron, D. Duneau, D.P. Hughes & F. Thomas, 2006. Parasite survives predation on its host. Nature 440: 756. Doi: 10.1038/440776a
Biron, D.G., L. Marché, F. Ponton, H.D. Loxdale, N. Galéotti, L. Renault, C. Joly & F. Thomas, 2005. Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach. Proceedings of the Royal Society B 272: 2117-2126. Doi: 10.1098/rspb.2005.3213